
Ionic radii are typically determined using measurements from the crystal lattice structures of ionic compounds.

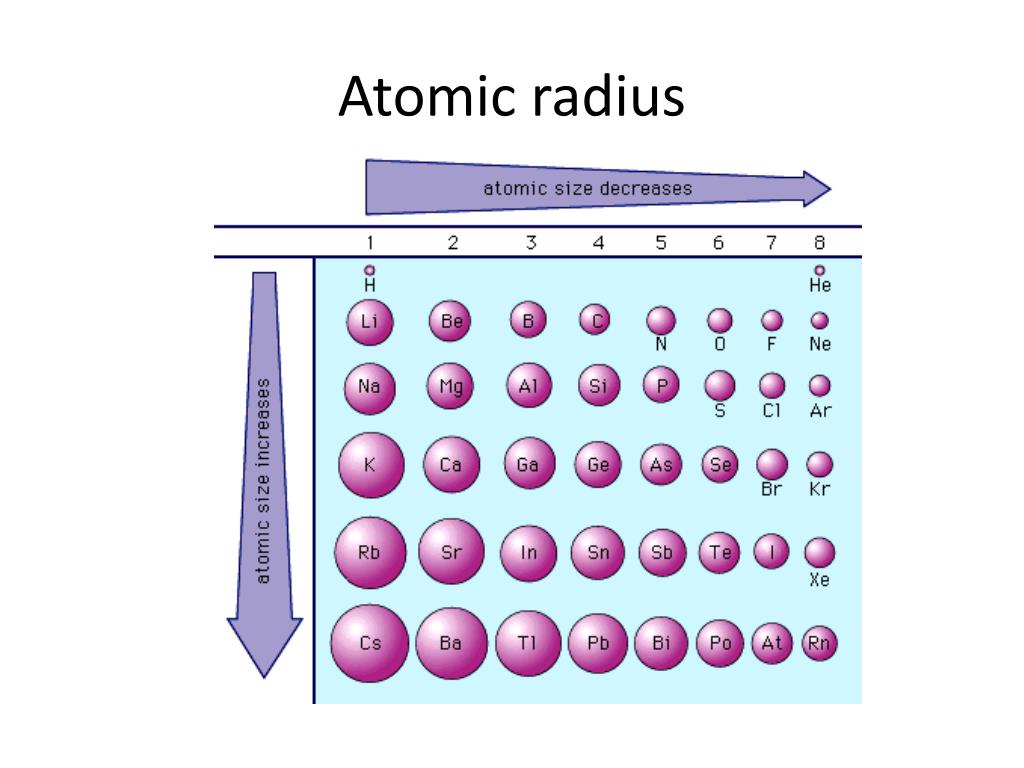
The ionic radius differs from the atomic radius in that the ionic radius is the radius of a charged species, and the atomic radius is the radius of a neutral atom. The atomic radii and effectively nuclear charges for the 2nd period elements is show below. We've now established that effective nuclear charge increases left to right across a period and see how it explains the corresponding decrease in atomic radius. Overall, as one proceeds from left to right across a period there is an increase in the number of protons in the nucleus while the number of shielding electrons remains constant leading to an increasing effective nuclear charge. Magnesium's valence electrons experience a higher effective nuclear charge which indicates a greater attraction to the nucleus explaining why it would have a smaller atomic radius (130pm for magnesium vs 154pm from sodium). Magnesium has 12 protons (Z = 12) and 10 core electrons (S = 10) and its effective nuclear charge is Sodium has 11 protons (Z = 11) and 10 core electrons (S = 10) and its effective nuclear charge is Sodium and magnesium are a good comparison to demonstrate the trend. It turns out that not all core electrons will contribute the same repulsion either as described in Slater's Rules, but the rough approximation being used here will be sufficient to explain the trend in atomic radius. This is not technically true but a decent approximation for our purposes as the repulsion from another valence electron should be considerable less than from a core electron. This methodology says that valence electrons don't experience repulsion from each other. For a valence electron the number of shielding electrons is simply equal to the number of core electrons. Where Z is the number of protons and gives the charge of the nucleus and S represents the number of 'shielding' or 'screening' electrons. The effective nuclear charge can be approximated by the following simplified formula: It accounts for both the attraction to the protons in the nucleus and the repulsion from the other electrons in the atom. The effective nuclear charge (abbreviated Z eff) is measure of the strength of the attraction of a valence electron to the nucleus. This trend in atomic radius is best understood in terms of the effective nuclear charge experienced by the valence electrons. But if we keep in mind that the atomic mass is concentrated in the nucleus whereas the atomic radius is related more to the size of the 'electron cloud' it can be understood that these trends do not need to align. Initially this seems counter-intuitive as this increase in radius is accompanied by a general decrease in atomic mass. Atomic radius increases right to left across a period on the periodic table.


 0 kommentar(er)
0 kommentar(er)
